At YJCPolymer, we specialize in the production of high-quality, medical-grade products that meet the strictest standards for patient safety and performance. One of our most recent successful collaborations involved the design and manufacturing of a series of components for medical devices, specifically the Medical Grade PVC Cuffed Endotracheal Anesthesia Throat Tube with Inner Cannula Fenestrated Tracheostomy Tube Parts. Our client, a leading healthcare manufacturer based in India, approached us with a challenging set of requirements and a need for precise, durable components for their life-saving medical devices.
In this case study, we will walk you through the challenges our team faced during production, how we worked directly with our client to address these issues, and how our expertise in molding, materials, and manufacturing processes helped us deliver the components on time, ensuring long-term satisfaction and a strong ongoing partnership.

The Initial Challenge: Meeting Strict Medical Device Standards
Medical-grade devices, particularly those used in anesthesia and respiratory support, must meet stringent standards for safety, performance, and biocompatibility. Our Indian client required PVC Cuffed Endotracheal Anesthesia Throat Tubes with an inner cannula and a fenestrated tracheostomy tube design. These parts are essential for facilitating airway management during anesthesia and tracheostomy procedures, and any failure in their production could lead to serious medical consequences.
Our client’s primary challenge was ensuring that every part was compliant with international medical standards, especially in areas like:
- Material Integrity: PVC must be flexible, durable, and biocompatible while withstanding high levels of sterilization.
- Precision in Molding: The design of the cuffed endotracheal tube, inner cannula, and fenestrated tracheostomy tube required extremely tight tolerances to ensure proper fit and functionality.
- Surface Finish: Parts used in medical applications must have smooth, non-abrasive surfaces to prevent irritation to the patient’s airways.
The client had already attempted production through other manufacturers, but the parts were consistently coming up short in these areas, leading to delays and additional costs. They needed a reliable partner to resolve these issues and bring the parts into compliance with their requirements.
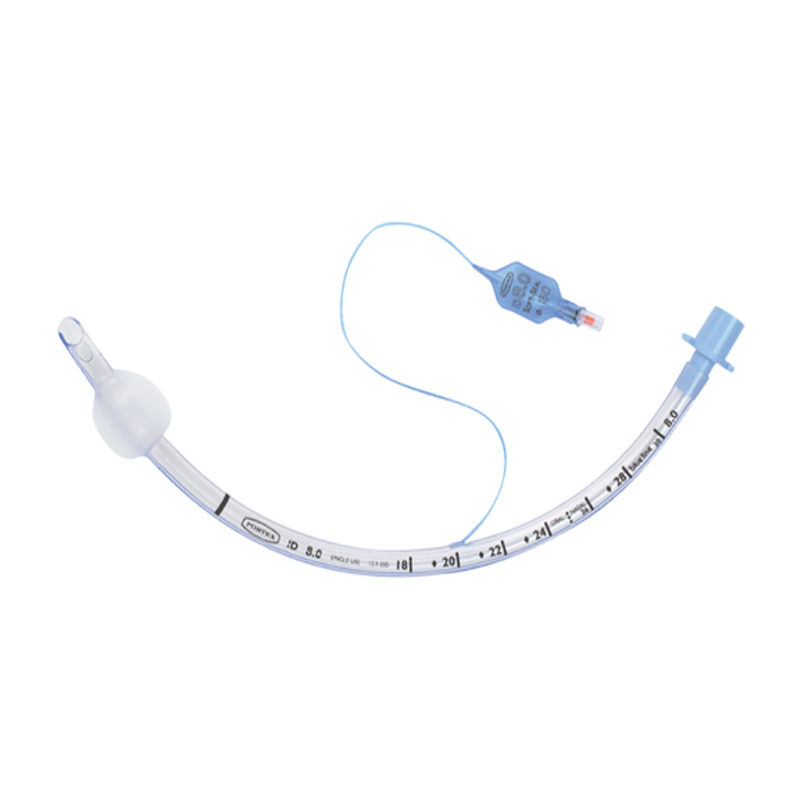
The Solution: Collaborative Approach with In-Depth Analysis
After initial discussions with the client, our team at YJCPolymer immediately set up a face-to-face meeting to understand the challenges in greater detail and develop a tailored solution. This direct communication was critical in addressing key concerns and ensuring that we were aligned with the client’s expectations. We followed a thorough, step-by-step approach to resolving the issues and ensuring that every part met the required specifications.
1. Mold Design and Manufacturing Analysis
One of the most pressing issues our client faced was mold design and production. The PVC Cuffed Endotracheal Anesthesia Throat Tube with Inner Cannula required precise dimensions to ensure the cuff sealed correctly and that the inner cannula could be easily removed or replaced without compromising the tube’s integrity.
Our in-house experts analyzed the existing mold designs and discovered that the tooling used by the client’s previous suppliers was not optimized for the intricate internal geometry of the tracheostomy tube. The mold needed to be modified to accommodate complex internal features, like the fenestrations in the cannula and the cuff mechanism.
To address this, we developed a custom mold design that would improve the production of the parts, focusing on:
- High Precision Tooling: We utilized CNC machining and electrical discharge machining (EDM) to refine the mold’s cavities and ensure precise fit and function.
- Advanced Mold Cooling Systems: To reduce cycle times and improve part quality, we implemented a specialized mold cooling system that would allow for uniform cooling, minimizing the risk of warping or dimensional inaccuracies.

2. Material Selection and Compatibility
Selecting the right material for medical-grade components is crucial for ensuring that the parts are safe, durable, and biocompatible. Our client originally selected standard PVC, but after reviewing their requirements, we recommended using medical-grade PVC, a material that is flexible, highly durable, and resistant to degradation under sterilization conditions.
To ensure biocompatibility, we sourced PVC that met the ISO 10993 standards, which evaluate the material’s safety for contact with human tissues. Our team also tested the material for resistance to gamma radiation sterilization, which is commonly used in medical device production.
3. Surface Treatment and Finish
One of the most critical factors in medical device manufacturing is the surface finish. The inner surface of the cuffed endotracheal tube and inner cannula must be smooth and non-abrasive to prevent damage to the patient’s airways. Additionally, the exterior surface needed to be free of any defects that could lead to discomfort or injury.
At YJCPolymer, we implemented a precision molding process that ensured the parts were free of flash, burrs, and imperfections. We also applied specialized coatings to improve the surface finish and prevent irritation, ensuring that the parts met the high hygiene standards required in medical environments.

4. Prototyping and Testing
With the updated mold design, material selection, and surface finish, we moved into the prototyping phase. We produced initial samples of the cuffed endotracheal tube and inner cannula, followed by a series of rigorous testing procedures:
- Fit and Function Tests: We tested the prototypes for correct fit and function, ensuring that the cuff inflated properly, the inner cannula could be inserted and removed smoothly, and the fenestrations provided optimal airflow.
- Biocompatibility Testing: We conducted biological testing on the final product to ensure that it met ISO 10993 standards for cytotoxicity and irritation.
Our client was highly impressed with the quality of the prototypes, and after a few minor adjustments, we moved forward to full-scale production.
Successful Partnership and Ongoing Collaboration
The collaboration between YJCPolymer and our client in India was a resounding success. By working directly with the client and analyzing every step of the production process, we were able to resolve the issues they had been facing with mold design, material compatibility, surface treatment, and testing. As a result, we delivered the final components on time, in compliance with all medical standards, and with the durability and functionality required for the intended use.
Our client was extremely satisfied with the results, and this successful project has led to a long-term partnership between YJCPolymer and the healthcare manufacturer. We continue to collaborate with them on additional medical-grade products, applying our expertise to ensure that all components meet the highest safety standards.

Conclusion: How We Can Help You Achieve Success in Medical Device Manufacturing
At YJCPolymer, we understand the critical nature of producing medical-grade components that meet rigorous standards for safety, functionality, and biocompatibility. By partnering with us, you gain access to our deep expertise in mold design, material selection, surface treatment, and biocompatibility testing, ensuring that your medical device parts are of the highest quality.

If you’re facing challenges in the production of medical-grade parts, whether it’s related to mold design, materials, or quality control, YJCPolymer is here to help. Contact us today to learn more about our capabilities and how we can support your project from design to production, ensuring that your products meet both regulatory requirements and customer expectations. We’re ready to work with you and turn your vision into a reality.